Isotope Geochemistry
Prof. Massimo D’Antonio | Prof. Valeria Di Renzo | Prof. Paola Petrosino | Prof. Vincenzo Morra | Prof. Leone Melluso | Dr. Ciro Cucciniello | Dr. Lorenzo Fedele
Dr. Ilenia Arienzo, PhD – INGV-OV, Naples
Dr. Federica Totaro, post-doc – DiSTAR
Dr. Carlo Pelullo, post-doc – INGV-OV, Naples
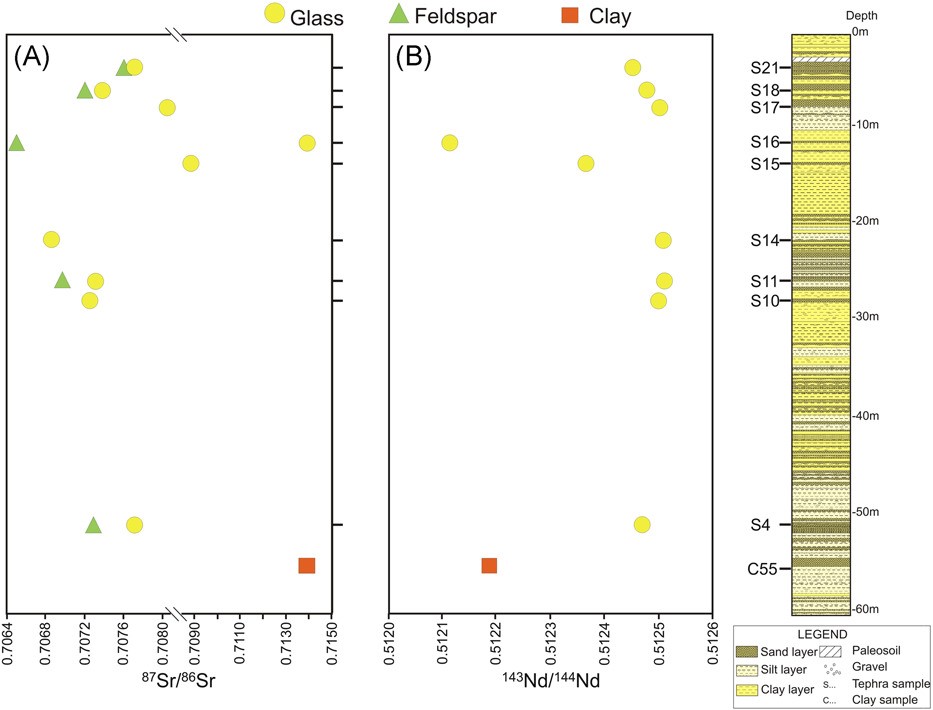 Isotopic chemostratigraphy of tephra samples collected from a drill-hole carried out in the San Gregorio Magno lacustrine basin (near Salerno, Southern Italy).
Isotopic chemostratigraphy of tephra samples collected from a drill-hole carried out in the San Gregorio Magno lacustrine basin (near Salerno, Southern Italy).
Isotopic chemostratigraphy of tephra samples collected from a drill-hole carried out in the San Gregorio Magno lacustrine basin (near Salerno, Southern Italy).
Isotope Geochemistry is the branch of knowledge that uses the isotopic composition of naturally occurring chemical elements to either perform absolute dating of geological material samples or trace geological processes.
The Radiogenic Isotope Geochemistry utilizes chemical elements generally having high atomic number, such as strontium, neodymium and lead. The isotopic composition of such elements varies as a consequence of their longer or shorter persistence in a “reservoir” of the Earth, that can be a portion of the mantle or crust. During such a permanence time, the radiogenic isotope of an element growths because of the radioactive decay of another, parent chemical element. For instance, 87Sr increases with time as a consequence of the radioactive decay of 87Rb.
The Stable Isotope Geochemistry utilizes the fractionation among the isotopes of a chemical element generally having low atomic number, such as hydrogen, boron, oxygen, nitrogen and sulphur. The isotopic fractionation consists in enrichment of a lighter isotope relative to a heavier isotope, and vice versa, of a chemical element, in the course of natural processes, such as: transformation of minerals during a metamorphic event; precipitation of secondary minerals during diagenesis or pedogenesis; water evaporation or condensation; organic matter generation through photosynthesis of chemosynthesis; open-system magmatic evolution.
Isotope Geochemistry provides efficient tools for investigating several geological processes, such as: assimilation of crustal rocks by a crystallizing magma; mixing among distinct magma batches; mixing among water masses of variable provenance; mixing among clasts of variable composition in a sedimentary basin. In volcanology, 87Sr/86Sr, 143Nd/144Nd, 206Pb/204Pb and d11B values allow drawing of isotope chemostratigraphies for the reconstruction, in conjunction with the variation of other geochemical parameters, of the evolution through time of the magmatic feeding system of a volcano, even during a single eruption. In petrology, the isotopic tracers, combined with trace element contents and ratios, allow quantitative modelling of magma source regions, as well as genesis and evolution processes of magmas in different geodynamic settings on Earth. Combining radiogenic and stable isotopes (see figure) allows discriminating between mantle source enrichment processes involving sedimentary components, from crustal contamination processes. Moreover, stable isotopes allow estimating the equilibrium temperature of magmatic, metamorphic and metallogenic processes.
Lastly, the isotopes of several metals allow tracing the effects of anthropogenic activities on both environment and man, such as: groundwater and soil pollution by industrial or agriculture wastewaters; soil pollution by aerosols containing tetraethyl lead from gasoline; pollution of human tissues by toxic metals, such as Pb, Cr and Cd. In the agro-food sector, radiogenic isotopes are employed since a few years as tracers for relating several food and beverages (cheeses, vegetables, wine, oil) to the territory where raw materials are produced, in order to shed light on possible food fraud.